Verified Clinical Trials and Remote and Virtual Clinical Trials. Preventing Professional Research Subjects With A Global Database Registry
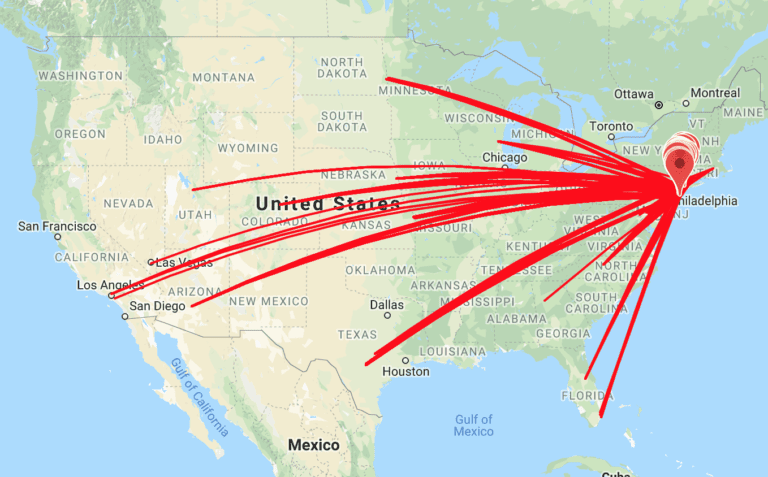
Conducting virtual clinical trials or remote clinical trials, or just a standard clinical trial? Verify your research subject’s eligibility and ensure they are not concurrently enrolling in multiple clinical trials at once. Verified Clinical Trials eliminates professional research subjects or duplicate research subjects. This can be accomplished completely off site with our newest technology. Avoid …