Research Sites Cooperate To Prevent Duplicate Subjects in CNS Clinical Trials By Using The Verified Clinical Trials Research Subject Registry
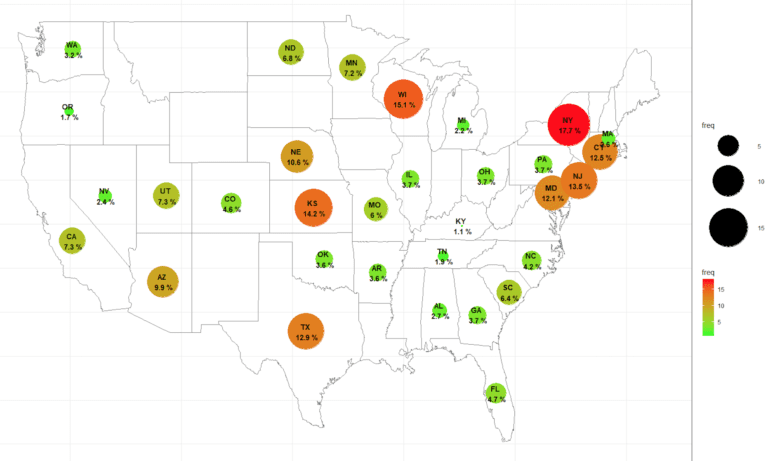
Duplicate subjects exist in most clinical trials to varying degrees. CNS clinical trials are especially prone to duplicate subject or over enrollment. The issue exists from phase 1 healthy volunteer studies to multiple phase 2/3 clinical trials across many therapeutic indications. Use of global research subject database registry such as Verified Clinical Trials will help …