Understanding Research Subjects Behaviors & Travel Habits: Duplicate Subjects In CNS Clinical Trials
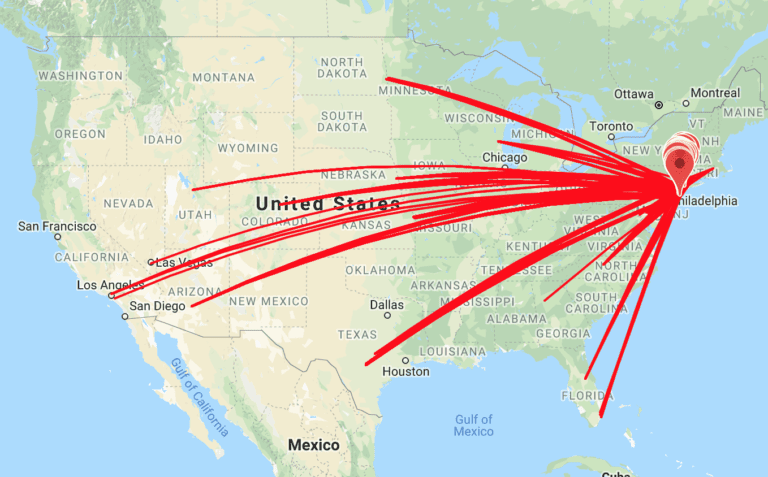
Verified Clinical Trials (VCT) presented a poster at the ASCP meeting in Phoenix May 2019, detailing travel habits and distances specifically in CNS clinical trials. Bottom line: In many instances, research subjects travel large distances to become duplicate subjects in CNS clinical trials. A research subject database is needed to prevent duplicate subjects and many …