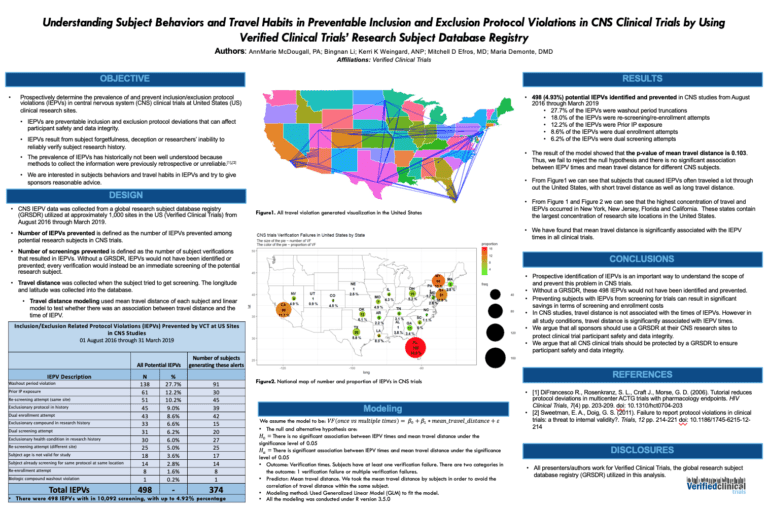
Verified Clinical Trials Sees Progress In The Understanding and Acceptance of Duplicate Subjects In Clinical Trials
We have seen tremendous progress within the clinical trials research industry in understanding the scope of the issue with duplicate subjects in clinical trials or professional research subjects over the past few years. Verified Clinical Trials (VCT) provides an easy to implement cost-savings approach to prevent duplicate subjects and other important protocol violations in your …