CNS clinical trials, especially psychiatry clinical trials are prone to having duplicate subjects in clinical trials. In studies that rely upon subjective end points, there exists the ability to provide false information in an effort to gain entry in to the study. Of course, these occurrences result in failed trials due to elevated placebo rates or issues with safety if the volunteer does in fact take the investigational product (IP) from multiple sites.
Use of the Verified Clinical Trials (VCT) global research subject registry has been quite effective at stopping these issues at the time of screening. The protections continue throughout the entire study. The Verified Clinical Trials network is expansive and covers multiple countries worldwide. Nearly every phase 1 unit across the United States and now in Europe and elsewhere. VCT protects many therapeutic indications but is especially focused on duplicate subjects in CNS clinical trials and duplicate subjects in psychiatry clinical trials.
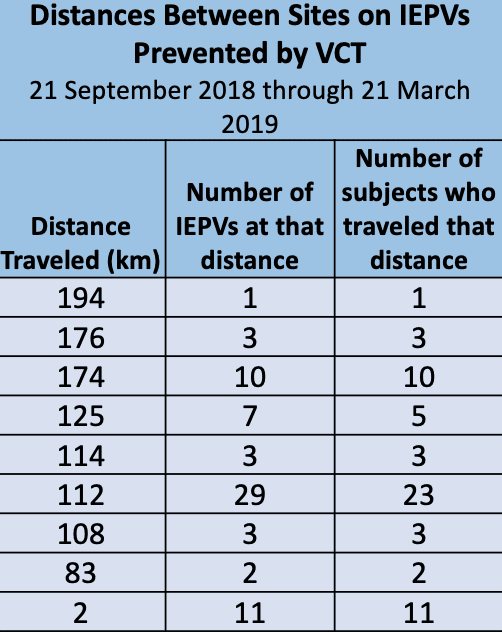
The data is rather impressive with as preventable protocol violations including duplicate or dual enrollment in clinical trials ranging higher than 10% in some instances. The National Institute of Health (NIH) uses VCT exclusively.