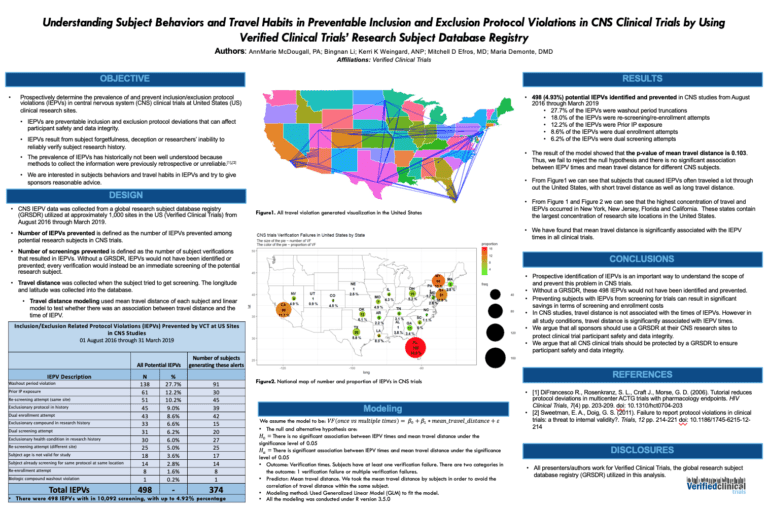
Verified Clinical Trials Offers Virtual Clinical Trial Solution To Prevent Co-Enrollment or Duplicate Subjects
Conducting virtual or remote clinical trials? Verify your research subjects eligibility and ensure they are not concurrently enrolling in multiple clinical trials at once. Avoid co-enrollment in clinical trials or duplicate subjects in clinical trials as well as protocol violations. Select better quality subjects for your trial so that you can meet her in points …