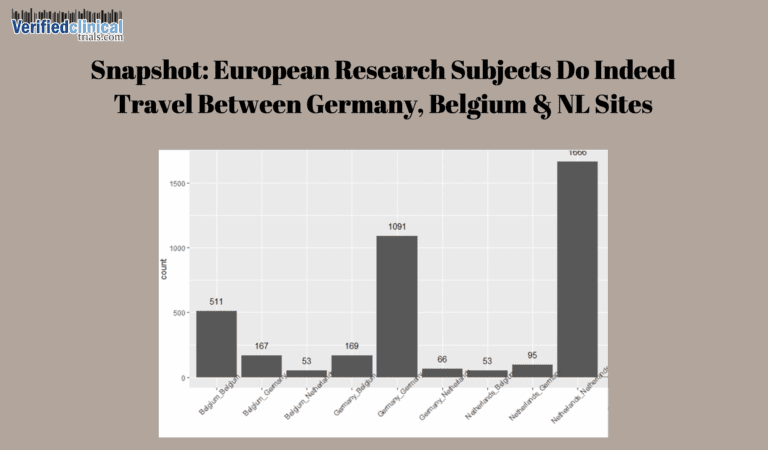
Duplicate Subjects in Clinical Trials Proactively Detected and Prevented By Verified Clinical Trials
There are many obstacles to the success of a clinical trial and duplicate research subjects, or professional research subjects are unfortunately one of them. Duplicate subjects in clinical trials results in a myriad of poor outcomes. Ultimately duplicate research subjects can result in a clinical trial failing to meet its endpoints. And now with decentralized …