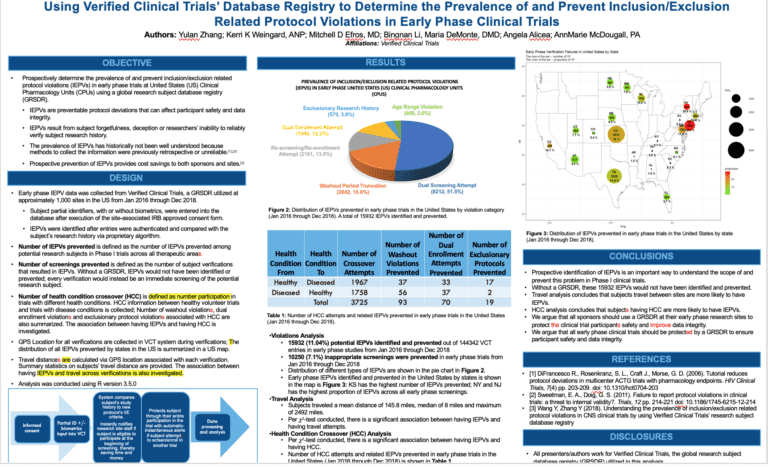
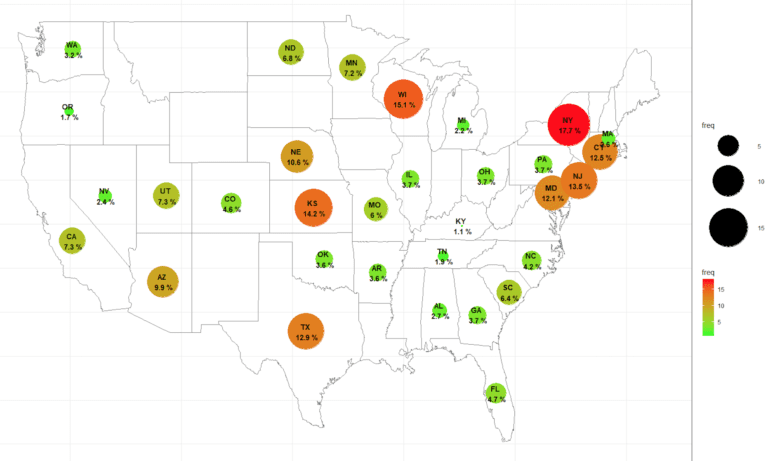
Duplicate subjects or professional research subjects are many times the silent killer in your clinical trials
Duplicate subjects in clinical trials result in increased placebo rates and poor quality data. This is oftentimes the silent killer of your study. www.verifiedclinicaltrials.com (VCT) is the global research subject database registry that is utilized by countless sites and sponsors worldwide. Duplicate subjects in clinical trials cause safety and dat quality issues. They drive up the …