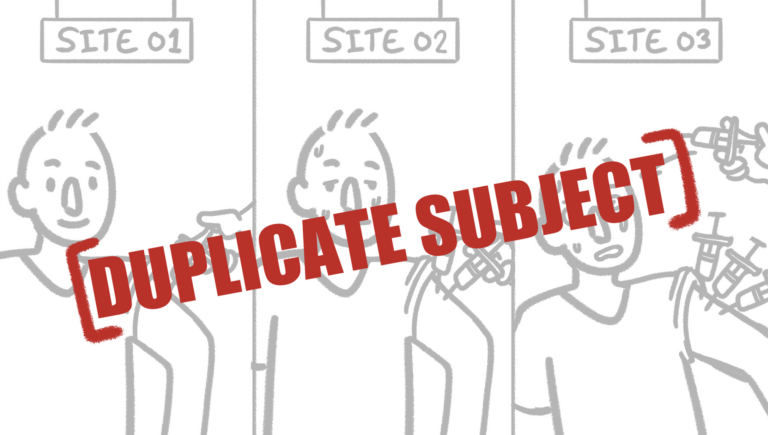
Preventing Duplicate Subjects in Clinical Trials: Verified Clinical Trials Leads the Way at ASCP Annual Meeting
The Verified Clinical Trials (VCT) team is excited to attend the 2025 ASCP Annual Meeting, currently taking place in Tucson, Arizona. As the premier event hosted by the American Society of Clinical Psychopharmacology, ASCP brings together global leaders advancing psychiatry and CNS (central nervous system) drug development—and VCT is proud to support that mission. Verified …