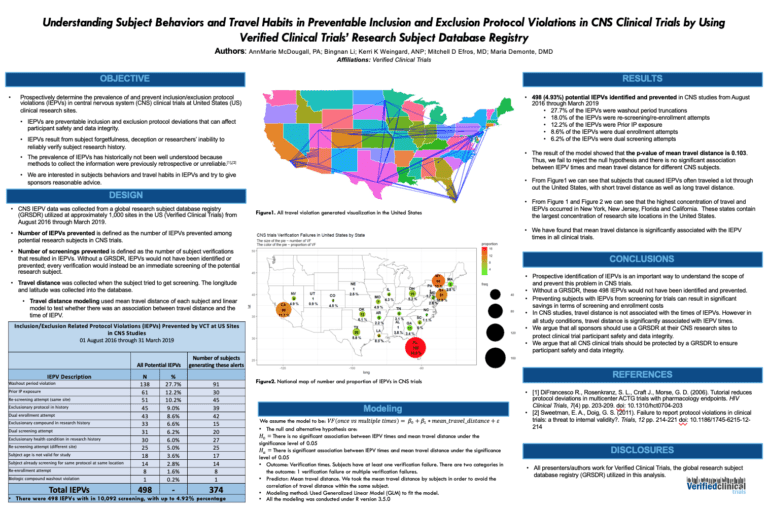
Subject Registries Reduce Duplicate Subjects Entering CNS Studies
Verified Clinical Trials (VCT) is the global research subject database registry utilized across all phases of clinical trials research to prevent duplicate subjects and other key protocol violations. Data shows that a research subject registry will detect and prevent duplicate subjects and other protocol violations. Up to 10% in CNS clinical trials and much higher …