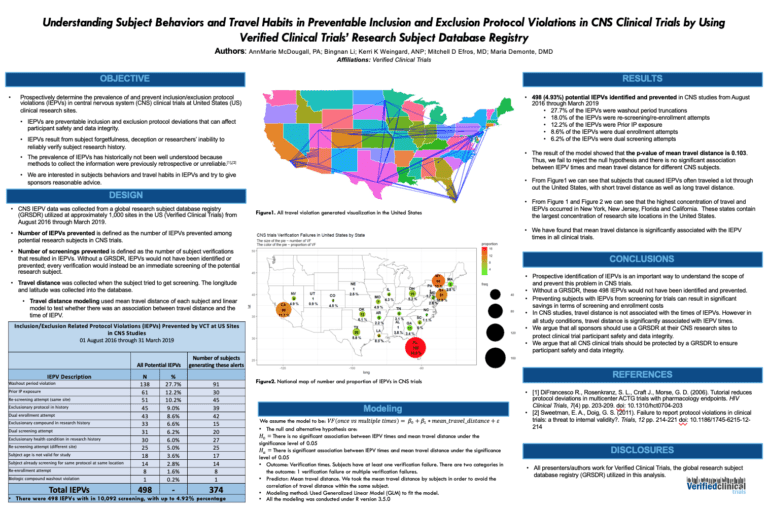
How to Avoid The Dangers of Duplicate Participants in Clinical Trials
The prevalence of duplicate participants in clinical trials poses significant risks, compromising the integrity of data and the safety of the patients involved. To delve into this matter, we spoke with Kerri Weingard, a seasoned veteran in the field and the co-founder of Verified Clinical Trials (VCT), who brings a wealth of knowledge and experience. In this …